Good Manufacturing Practice (GMP) is a system for ensuring that products are consistently produced and controlled according to quality standards. It is designed to minimize the risks involved in any production that cannot be eliminated through testing the final product.
Practices are recommended with the goal of safeguarding the health of consumers and patients as well as producing good quality food, medicine, medical devices, or active pharmaceutical products.
GMP covers all aspects of production from the starting materials, premises and equipment to the training and personal hygiene of staff.
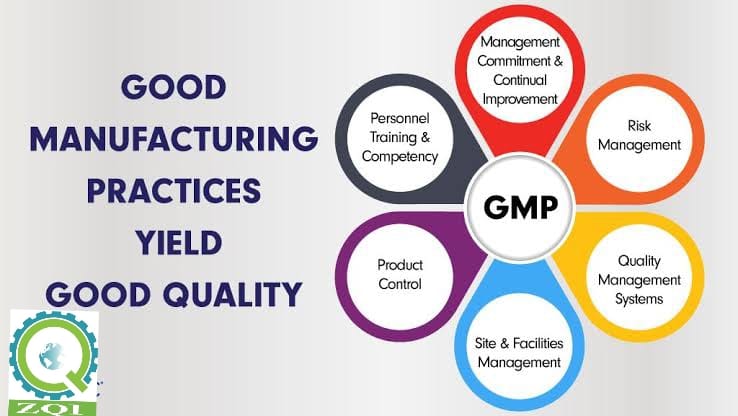
The key benefits to GMP certification:
i) Prove organization’s management capabilities in product quality, safety assurance
ii) Enable employees to develop good production / operations habits
iii) Reduce safety risk in product quality and safety
iv) Timely detect production and management problems, reduce cost
v) Better understand and comply with the relevant laws and regulations
vi) Enhance the international credibility and public image
vii) Increase customer’s long-term confidence in the enterprise